The landscape of antibiotic development and regulation in the United States and Europe, alongside evolving industry incentive strategies
The Petauri Evidence team will be presenting this research at ISPOR Europe in Glasgow, in Poster Session 5 on Wednesday 12th November.
View poster's referencesIn this short video, Benjamin Bradbury (Health Economist, Petauri Evidence) introduces the research:
Poster introduction:
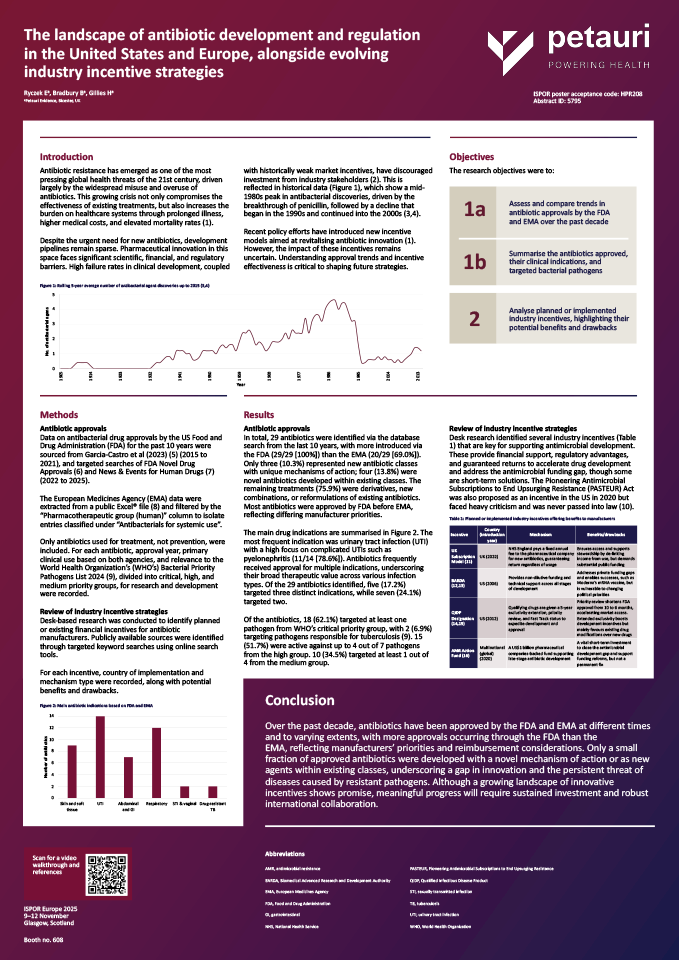
Antibiotic resistance has emerged as one of the most pressing global health threats of the 21st century, driven largely by the widespread misuse and overuse of antibiotics. This growing crisis not only compromises the effectiveness of existing treatments, but also increases the burden on healthcare systems through prolonged illness, higher medical costs, and elevated mortality rates (1).
Despite the urgent need for new antibiotics, development pipelines remain sparse. Pharmaceutical innovation in this space faces significant scientific, financial, and regulatory barriers. High failure rates in clinical development, coupled with historically weak market incentives, have discouraged investment from industry stakeholders (2). This is reflected in historical data, which show a mid-1980s peak in antibacterial discoveries, driven by the breakthrough of penicillin, followed by a decline that began in the 1990s and continued into the 2000s (3,4).
Recent policy efforts have introduced new incentive models aimed at revitalising antibiotic innovation (1). However, the impact of these incentives remains uncertain. Understanding approval trends and incentive effectiveness is critical to shaping future strategies.
Research objectives:
1a) Assess and compare trends in antibiotic approvals by the FDA and EMA over the past decade
1b) Summarise the antibiotics approved, their clinical indications, and targeted bacterial pathogens
2) Analyse planned or implemented industry incentives, highlighting their potential benefits and drawbacks
Please complete the form below to download the full poster:
Back to ISPOR Europe Virtual Booth
To help save the planet and to save you from carrying a tonne of literature around the exhibition hall, all our resources are available virtually.
Poster references:
- WHO. Antimicrobial resistance. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed: September 2025.
- Thomas D, Wessel C. The State of Innovation in Antibacterial Therapeutics. BIO Industry Analysis. 2022. Available from: https://www.bio.org/sites/default/files/2022-02/The-State-of-Innovation-in-Antibacterial-Therapeutics.pdf. Accessed: September 2025.
- Sepkowitz KA. One hundred years of Salvarsan. N Engl J Med. 2011;365(4):291-3.
- Powers JH. Antimicrobial drug development–the past, the present, and the future. Clin Microbiol Infect. 2004;10 Suppl, 4:23-31.
- García-Castro M, et al. Approved antibacterial drugs in the last 10 years: from the bench to the clinic. Explor Drug Sci. 2023;1:180-209.
- FDA. Novel Drug Approvals at FDA. 2025. Available from: https://www.fda.gov/drugs/development-approval-process-drugs/novel-drug-approvals-fda. Accessed: September 2025.
- FDA News & Events for Human Drugs. 2025. Available from: https://www.fda.gov/drugs/news-events-human-drugs. Accessed: September 2025.
- EMA. Download medicine data. 2025. Available from: https://www.ema.europa.eu/en/medicines/download-medicine-data. Accessed: September 2025.
- WHO. WHO Bacterial Priority Pathogens List 2024. 2024. Available from: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1. Accessed: September 2025.
- Access Campaign. The PASTEUR Act is not the way for the US government to address antimicrobial resistance. 2024. Available from: https://msfaccess.org/pasteur-act-not-way-us-government-address-antimicrobial-resistance. Accessed: September 2025.
- UK Parliament. “Netflix” for antimicrobials: The Antimicrobial Products Subscription Model. 2024. Available from: https://commonslibrary.parliament.uk/netflix-for-antimicrobials-the-antimicrobial-products-subscription-model/. Accessed: September 2025.
- US Department of Health and Human Services. About BARDA. 2025. Available from: https://medicalcountermeasures.gov/barda. Accessed: September 2025.
- IFP. Why BARDA Deserves More Funding. 2022. Available from: https://ifp.org/why-barda-deserves-more-funding/. Accessed: September 2025.
- US Department of Health and Human Services. Qualified Infectious Disease Product Designation – Questions and Answers Guidance for Industry. 2021. Available from: https://www.fda.gov/media/148480/download. Accessed: September 2025.
- Darrow JJ, Kesselheim AS. Incentivizing Antibiotic Development: Why Isn’t the Generating Antibiotic Incentives Now (GAIN) Act Working? Open Forum Infect Dis. 2020;7(1):ofaa001.
- IFPMA. AMR Action Fund. 2024. Available from: https://www.ifpma.org/initiatives/amr-action-fund/. Accessed: September 2025.
