Shared Immune Targets in Neurodegenerative Diseases

Could patients with neurodegenerative disorders, such as Alzheimer’s disease (AD) or Parkinson’s disease (PD), be cured? Despite the increasing prevalence of these conditions, there are currently no treatments that have been shown to fully halt or reverse their progression. However, a growing body of evidence indicates that the immune system plays a significant role in the onset and progression of these disorders. As a result, identifying shared immune targets may offer a promising therapeutic strategy capable of addressing a broad range of neurodegenerative diseases.
Neurodegenerative diseases represent a diverse group of neurological disorders marked by impairments in cognition, memory, behavior, sensory, and motor functions.1,2 AD and PD are among the most prevalent neurodegenerative diseases, with increasing incidence rates globally.1,3,4 AD, the leading cause of dementia, affects an estimated 27 million people worldwide. Its pathology involves the accumulation of amyloid β plaques, formation of neurofibrillary tangles, protein tau hyperphosphorylation, and chronic neuroinflammation.5-8 Clinically, AD manifests as progressive cognitive decline, memory loss, and irreversible behavioral disturbances.3 PD is the second most common neurodegenerative disorder, affecting approximately 12 million patients globally. Around 1% of individuals over the age of 60 years are affected. PD is characterized by the aggregation of α-synuclein, which disrupts dopaminergic neurons and contributes to neurodegeneration.4,9–11 Clinical manifestations include motor symptoms, such as tremor, stiffness, slowness, and postural instability. Additionally, patients with PD experience a range of nonmotor symptoms, including sleep dysfunction, olfactory loss, psychiatric disturbances, and cognitive impairment.12
The immune system plays a crucial role in maintaining health and defending the body against pathogens and disease through a complex network of innate and adaptive immune components.1 Innate immunity is present from birth and serves as the body’s first line of defense against external threats. It responds rapidly and non-specifically to pathogens.1,13 In contrast, adaptive immunity provides a more targeted and specific defense that develops over time against foreign antigens, those not recognized as self. It has an immunological memory, allowing it to recognize and respond more effectively to previously encountered antigens.
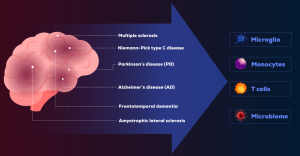
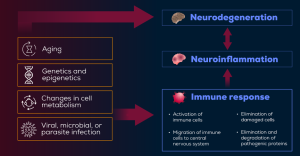
The immune system is increasingly recognized as a key player in the pathogenesis of neurodegenerative diseases.14 These disorders frequently involve immune dysregulation and neuroinflammation, with overlapping mechanisms contributing to disease progression. Key pathological indicators include protein aggregation, defects in the endosomal-lysosomal network, mitochondrial dysfunction, oxidative stress, and clearance of damaged proteins.15 Immune cells such as microglia, monocytes, T cells, and even the microbiome have emerged as common targets in the immunopathology of neurodegenerative diseases (Figure).14 Below, we will walk through the current evidence supporting these key players in the immune system as potential therapeutic targets.
Figure. Interaction Between Neurodegenerative Diseases, Immune Response, Neuroinflammation, and Neurodegeneration16
Microglia
Microglia play a critical role in the pathophysiology of various neurological disorders.14,15 These immune cells monitor the microenvironment and respond to disease or injury. Microglia are typically categorized into 2 types: neurodegenerative and homeostatic.14,17 While homeostatic microglia are essential for maintaining brain health, neurodegenerative microglia are associated with disease states. In AD, studies suggest that homeostatic microglia shift to a disease-associated type as the disease progresses.14,18
Early in AD, these immune cells may play a protective role, but over time, they adopt a harmful phenotype that causes neuronal damage. As microglia contribute to the pathogenesis of AD, they appear to be a promising therapeutic target. Because of their changing phenotype during the disease course, a treatment that controls microglial type may be beneficial. Further therapeutic strategies could focus on modulation of microglia, lipid metabolism, and lysosome (organelles responsible for the breakdown of various molecules) function.14
In PD, activated microglia are found in the brain regions where dopaminergic neurons are lost. Studies in animals have shown that α-synuclein activate and bind microglia, resulting in reduced phagocytosis, a process of engulfing and digesting harmful substances, and impaired α-synuclein clearance. Also, α-synuclein is associated with the inflammatory microglial response. Potential treatment options for PD may include microglial replacement therapy, control of microglial activation, and promotion of phagocytosis for α-synuclein clearance.14,19
Monocytes
Monocytes are another key component of the innate immune system. They are associated with phagocytosis, antimicrobial defenses, regulation of transendothelial migration, apoptosis, and wound healing.
In AD, monocytes exhibit inflammatory properties and express pro-inflammatory signals. Moreover, these immune cells are associated with impairment of phagocytosis, decreased expression and activity of lysosomal enzymes, and diminished degradation of Aβ1-42 peptide.
In PD, monocyte levels are elevated compared to healthy individuals, and some may contribute to dopaminergic neuron death. Moreover, monocytes bind α-synuclein and induce additional inflammation, contributing to impaired α-synuclein clearance. Later disease onset and shorter duration were associated with greater proliferation of monocytes. Potential therapeutic strategies may include targeting pathogenic monocytes, promoting reparative phenotypes, modulating monocyte function by nanoparticles or monoclonal antibodies, or transferring monocytes generated ex vivo.14
T cells
T cells are a subset of white blood cells that originate in the bone marrow.13,14 They are components of the adaptive immune system that recognize specific antigens via T-cell receptors presented by infected cells, thus protecting the body from illness. T lymphocytes are categorized into helper T (TH) cells (CD4+) managing immune response, cytotoxic T cells (CD8+) eliminating infected cells, and regulatory T (Treg) cells that prevent excessive inflammation.13,14
In AD, naïve T cells are decreased, whereas effector memory and CD4+ terminally differentiated effector cells are increased. The numbers of pro-inflammatory TH cells are elevated. Levels of activated cytotoxic CD8+ cells are increased in patients with mild AD or mild cognitive impairment. Studies have demonstrated that Treg cells have a beneficial function in AD, where they are associated with improved cognition and reduced amyloid β deposition. Depletion of Treg accelerates cognitive deficits caused by amyloid β deposition.14
In PD, reactivity of T cells to α-synuclein has been linked to worse clinical outcomes. CD4+ and Treg cell levels are lower in patients with PD. Dysregulation of Treg cells was linked to cognitive decline. Therapeutic approaches in neurodegenerative diseases should utilize Treg cells to diminish inflammation. Treatment involving Treg cells may include autologous and allogenic Treg cell therapy, chimeric antigen receptor (CAR) T cells, monoclonal antibodies, and small molecules that bind to Treg cells.14
Gut Microbiome
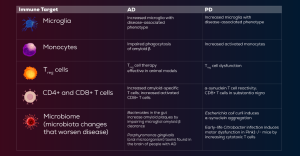
The gut microbiome influences both innate and adaptive immunity. Research involving microbiota transfers has shown that the microbiome contributes to neurodegenerative diseases by modulating the immune system. Microbiome depletion may be beneficial in both AD and PD. The mechanisms underlying these benefits include a reduction in proinflammatory T cells, an increase in Treg cells, and suppression of inflammatory microglia. Although microbiome-targeted therapies are still in the early stages of development, potential strategies include fecal microbiota transplantation, probiotics, dietary interventions, and gut-derived metabolites. Broad-spectrum antibiotic use is not recommended due to its lack of specificity. However, selective depletion of clearly defined pathogenic bacteria with bacteriophage-based approaches may offer favorable results (Table).14,15
Table. Evidence for Involvement of Immune Targets in Neurodegenerative Diseases14,20
Other neurological diseases that share immune targets—microglia, monocytes, T cells, microbiome—with AD and PD include multiple sclerosis and amyotrophic lateral sclerosis. Immune targets investigated in frontotemporal dementia and Niemann-Pick type C disease include microglia, macrophages, Treg cells, and the complement system. Stroke was found to be linked to effector T cells and activated microglia.14,15,21
Research continues to show that many neurodegenerative diseases share common pathological pathways, particularly those involving neuroinflammation and immune system dysregulation. This connection suggests the presence of shared immune targets across various conditions. Targeting these biomarkers could facilitate the development of pharmacological therapies that address multiple neurodegenerative diseases and may ultimately lead to effective treatments—or even cures—for these debilitating disorders.
References
- Huang Y, Zhang G, Li S, Feng J, Zhang Z. Innate and adaptive immunity in neurodegenerative disease. Cell Mol Life Sci. 2025;82(1):68.
- Wilson DM 3rd, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186(4):693-714.
- Kosyreva AM, Sentyabreva AV, Tsvetkov IS, Makarova OV. Alzheimer’s disease and inflammaging. Brain Sci. 2022;12(9):1237.
- Pardo-Moreno T, García-Morales V, Suleiman-Martos S, Rivas-Domínguez A, Mohamed-Mohamed H, Ramos-Rodríguez JJ, et al. Current treatments and new, tentative therapies for Parkinson’s disease. Pharmaceutics. 2023;15(3):770.
- Christensen A, Pike CJ. Menopause, obesity and inflammation: interactive risk factors for Alzheimer’s disease. Front Aging Neurosci. 2015;7:130.
- Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31-55.
- Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590.
- Silva MVF, Loures CMG, Alves LCV, de Souza LC, Borges KBG, Carvalho MDG. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(1):33.
- Foltynie T, Bruno V, Fox S, Kühn AA, Lindop F, Lees AJ. Medical, surgical, and physical treatments for Parkinson’s disease. Lancet. 2024;403(10423):305-324.
- Luo Y, Qiao L, Li M, Wen X, Zhang W, Li X. Global, regional, national epidemiology and trends of Parkinson’s disease from 1990 to 2021: findings from the Global Burden of Disease Study 2021. Front Aging Neurosci. 2025;16:1498756.
- Agnello L, Ciaccio M. Neurodegenerative Diseases: From molecular basis to therapy. Int J Mol Sci. 2022;23(21):12854.
- Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323(6):548-560.
- Park JE, Kim DH. Advanced immunomodulatory biomaterials for therapeutic applications. Adv Healthc Mater. 2025;14(5):e2304496.
- Weiner HL. Immune mechanisms and shared immune targets in neurodegenerative diseases. Nat Rev Neurol. 2025;21(2):67-85.
- De Marchi F, Munitic I, Vidatic L, Papić E, Rački V, Nimac J, et al. Overlapping neuroimmune mechanisms and therapeutic targets in neurodegenerative disorders. Biomedicines. 2023;11(10):2793.
- Garmendia JV, De Sanctis CV, Das V, Annadurai N, Hajduch M, De Sanctis JB. Inflammation, autoimmunity and neurodegenerative diseases, therapeutics and beyond. Curr Neuropharmacol. 2024;22(6):1080-1109.
- Butler R, Bradford D, Rodgers KE. Analysis of shared underlying mechanism in neurodegenerative disease. Front Aging Neurosci. 2022;14:1006089.
- Mason HD, McGavern DB. How the immune system shapes neurodegenerative diseases. Trends Neurosci. 2022 Oct;45(10):733-748.
- Morris HR, Spillantini MG, Sue CM, Williams-Gray CH. The pathogenesis of Parkinson’s disease. Lancet. 2024;403(10423):293-304.
- Amor S, Woodroofe MN. Innate and adaptive immune responses in neurodegeneration and repair. Immunology. 2014 Mar;141(3):287-91.
- Gendelman HE, Mosley RL. A perspective on roles played by innate and adaptive immunity in the pathobiology of neurodegenerative disorders. J Neuroimmune Pharmacol. 2015;10(4):645-50.

Share