What is driving the increase in terminated NICE appraisals?
The Petauri Evidence team will be presenting this research at ISPOR Europe in Glasgow, in Poster Session 5 on Wednesday 12th November.
In this short video, Helena Grant (Senior Analyst – Global Pricing and Market Access, Petauri Evidence) introduces the research:
Poster introduction:
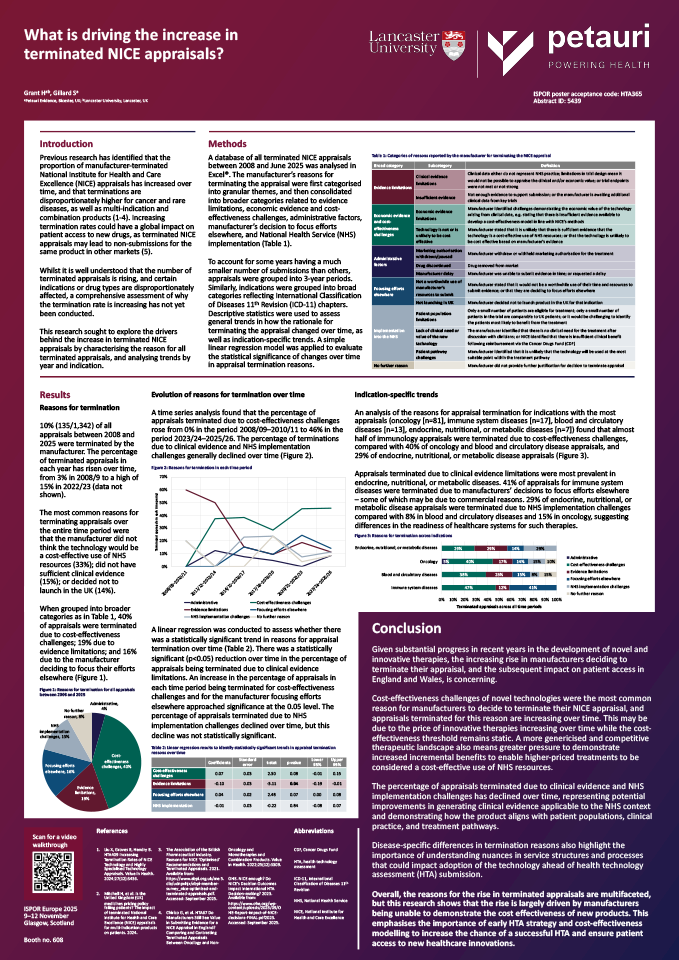
Previous research has identified that the proportion of manufacturer-terminated National Institute for Health and Care Excellence (NICE) appraisals has increased over time, and that terminations are disproportionately higher for cancer and rare diseases, as well as multi-indication and combination products. Increasing termination rates could have a global impact on patient access to new drugs, as terminated NICE appraisals may lead to non-submissions for the same product in other markets.
Whilst it is well understood that the number of terminated appraisals is rising, and certain indications or drug types are disproportionately affected, a comprehensive assessment of why the termination rate is increasing has not yet been conducted.
This research sought to explore the drivers behind the increase in terminated NICE appraisals by characterising the reason for all terminated appraisals, and analysing trends by year and indication.
Please complete the form below to download the full poster:
Back to ISPOR Europe Virtual Booth
To help save the planet and to save you from carrying a tonne of literature around the exhibition hall, all our resources are available virtually.
