Implementation of Cost-Effectiveness Analyses in Japan’s Health Technology Assessment Process

Japan’s evolving approach to market access includes a formal cost-effectiveness analysis (CEA) requirement for certain new medicines and medical devices. Introduced as part of a broader health technology assessment (HTA) framework in 2019, this process presents an additional hurdle for these products.
Here, Heather Wellam (Senior Consultant – Market Access, Petauri Evidence) explains the CEA process in Japan and explores the challenges and opportunities that the route offers for those looking to achieve market access:
- 5 quick questions on the cost-effectiveness process in Japan
- How we got here – the history of cost-effectiveness analysis in Japan
- Where we are now – the route to reimbursement
- The detail – calculating drug prices in Japan
- Challenges and opportunities
Editorial note – Originally published in March 2023 on our legacy Mtech Access website, this article received strong interest and positive feedback from clients. It quickly became one of our most-read pieces, so we’re pleased to share an updated version with you today, republished by Petauri Evidence (formerly Mtech Access and Delta Hat) in July 2025 on the Petauri website.
5 FAQs on the cost-effectiveness process in Japan
1) Who conducts the assessment of the CEA?
The Center for Outcomes Research and Economic Evaluation for Health (C2H) (1).
2) What is the aim of the CEA assessment?
To reduce expenditure and increase efficiency within the healthcare system by using CEA to evaluate the appropriateness of the price set by the Central Social Insurance Medical Council (Chuikyo), and, depending on the outcome of the assessment, to apply an upward or downward price adjustment (1).
3) What is the timeline?
If applicable, the assessment is conducted after a drug is listed on the National Health Insurance (NHI) drug standard. Selected manufacturers must submit a CEA to the C2H within 9 months, and the C2H takes approximately 6 months to complete their review. The total timeline is approximately 15 months (2).
4) What is the methodology?
The CEA system is based on the incremental cost-effectiveness ratio (ICER): cost per quality-adjusted life year (QALY) gained (see more).
C2H has published guidelines for manufacturers to support their CEA submission, including how to define the perspective, target population, comparator, and benefits. The guidelines also provide direction on methods for analysis, including calculating the ICER, determining time horizon, choice of outcome measure, and appropriate sources of clinical data, and calculating healthcare costs, productivity loss, discounting, modelling, and handling uncertainty (3).
5) How is the price adjusted after assessment?
Price adjustment rates are based on stepwise ICER thresholds and are applied to the launch premiums (if applicable) and to the operating profit portion of the NHI list price (jump to Calculating Drug Prices in Japan).
The History of CEA in Japan
In Japan, CEA for the purpose of public drug listing has been permitted by the Ministry of Health, Labour and Welfare (MHLW) since 1992. However, the data submission was voluntary, with little guidance given. As the data did not influence medicine pricing or listing, few pharmaceutical manufacturers submitted a CEA (1).
In 2012, the National Institute of Public Health (NIPH) founded the C2H . In 2016, this committee introduced mandatory CEAs on a trial basis for a small number of products.
After a series of consultations to refine the methodology, a consensus was reached, and a new CEA process was formally implemented in April 2019 (1).
CEA – The Route to Reimbursement in Japan
For selected products, the CEA process adds several steps to achieve market access. In addition to the standard process demonstrated in Steps 1 and 2 below, selected products are required to complete Steps 3–6 before their price is finalised.
Abbreviations: C2H, Center for Outcomes Research and Economic Evaluation for Health; CEA, cost-effectiveness analysis; Chuikyo, Central Social Insurance Medical Council; NHI, National Health Insurance; PMDA, Pharmaceuticals and Medical Devices Agency.
Step 1: Marketing Authorisation
A new product entering the Japanese market must first gain marketing authorisation from the Pharmaceuticals and Medical Devices Agency (PMDA).
Step 2: Reimbursement and listing on NHI drug standard
Following marketing authorisation, pharmaceutical companies must next apply to the Chuikyo to be listed on the NHI drug standard. The NHI drug standard lists the prices of drugs that are publicly reimbursed by the MHLW.
The reimbursed drug price is determined using one of two different pricing algorithms: the ‘similar efficacy comparison method’ or the ‘cost calculation (cost plus) method’. Premiums are also applied in certain circumstances. Jump to Calculating Drug Prices in Japan for more detail.
Once the product has been listed on the NHI drug standard, it is reimbursed under the public insurance-covered healthcare system. Historically, this was the full reimbursement process and, for some products, this is still the final hurdle to reimbursement.
However, since the inauguration of the CEA system in 2019, there are now additional market access hurdles for a subset of products.
Step 3: Cost-effectiveness assessment selection
Chuikyo then selects certain medicines and medical devices for cost-effectiveness analysis. Products are selected for assessment using a five-tier classification system. This is principally based on predicted annual peak sales and degree of innovation (premium). Products for rare diseases, haemophilia, and human immunodeficiency virus (HIV) are excluded. See Table 1 below:
Step 4: Preparation and submission of CEA to C2H
If a product is selected, then the pharmaceutical or medical device company must prepare a CEA. A reputable market access and health economics and outcomes research (HEOR) consultancy, like Petauri Evidence, is often best equipped to support companies with this step.
Step 5: Review and appraisal of CEA
The CEA is submitted to C2H for review and appraisal.
Step 6: Price re-evaluation & NHI standard list price adjustment
Chuikyo use this assessment to re-evaluate and, if necessary, adjust the NHI standard list price.
Calculating Drug Prices in Japan
When there is a comparable drug with the same indication, the ‘similar efficacy comparison method’ is used. Here the daily drug price of the new drug is matched to the daily drug price of the existing drug to ensure fair competition. If the new drug is evaluated as ‘superior’ then a premium may be awarded.
There are premiums for innovation, usefulness, marketability, paediatric indications, and treatment of severe illness (See Table 2).
When there is not a comparable drug with the same indication, the ‘Cost calculation (cost plus) method’ is used. Here, production costs, administrative costs, overheads, and operating profit are summed to determine a reimbursed price.
Medicines pricing algorithm in Japan: Cost calculation (cost plus) method
Once again, premiums are awarded if the drug is proven to be highly useful, as outlined in Table 2 below.
Table 2: Premiums that can be awarded to drugs if certain criteria are met
Japan is the first market to use a stepwise ICER criteria for determining the repricing rates.
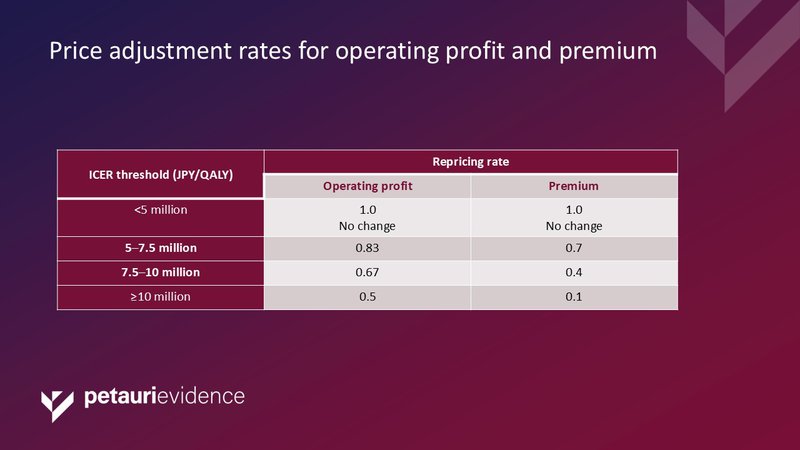
Table 3: Price adjustment rates for operating profit and premium
Abbreviations: QALY, quality-adjusted life year.
Some analysts have asked whether the ICER cut-offs of JPY <5 million/QALY, JPY 5–7.5million/QALY, JPY 7.5–10 million/QALY, and JPY ≥10 million/QALY are appropriate. The values were based on historic willingness-to-pay (WTP) surveys, and some challenge whether WTP is the most appropriate metric. Interestingly, the JPY <5 million threshold is higher than the threshold set by the UK’s National Institute for Health and Care Excellence (NICE) (£20,000), but lower than the US’s Institute for Clinical and Economic Review ($50,000).
Challenges and Opportunities with the CEA
For the subset of products that are selected for assessment, the development of a CEA is an additional hurdle to market access in Japan and may result in delays or barriers to patients receiving access to new interventions. Firstly, companies will need to assess whether their product will be selected to undergo a CEA. Next, they must ensure that all the relevant domestic inputs are available, and that they invest in the internal capabilities to submit the analysis in line with MHLW requirements.
Acquiring higher premiums through the Chuikyo pricing methodology has been a longstanding challenge for companies launching in Japan. The new system applies an increased pricing pressure and a risk of downward list price adjustment shortly after launch. The possibility of losing up to 90% of the drug premium and 50% of the operating profit within 1.5 years of launch undermines the innovation and usefulness reward, and may make Japan a less attractive market to manufacturers.
The repricing system is also anticipated to have a wider impact on the competitive set, with similar pricing adjustments expected to be applied.
Some critics of the system suggest that it has an over-reliance on the ICER and that other important factors are being overlooked. For example, disease severity, unmet therapeutic need, patient convenience, and societal impact.
The Japanese CEA system is in its infancy, and is likely to evolve, either through expansion of the scope of products assessed or in its potential remit. For example, if the CEA system was used in the future to determine reimbursement, this would create inevitable delays in the listing of a new product and will require more consideration from manufacturers of their launch priorities.
Support from Petauri Evidence
If you’re looking for insight and strategic advice on market access and reimbursement strategy across different markets, we can help. Email evidence@petauri.com to begin exploring your target markets with our global market access experts.
References
- Health NIoP. Application of cost-effectiveness in Japan. C2H. September 2022. Available from https://c2h.niph.go.jp/en/assessment/application/. Accessed July 2025.
- Health NIoP. Roles of C2H in cost-effectiveness evaluation. 2022. Available from https://c2h.niph.go.jp/en/assessment/roles/. Accessed July 2025.
- Health C. Guidelines for Preparing Cost Effectiveness Evaluation to the Central Social Insurance Medical Council version 30.0. CSIMC. 2022. Available from https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed July 2025.
- MHLW. Full scale introduction of cost-effectiveness evaluations in Japan. 2019. Available from https://c2h.niph.go.jp/tools/system/overview_en.pdf. Accessed July 2025.
A version of this insights piece was originally published in March 2023 on the Mtech Access website. Petauri Evidence (formerly Mtech Access and Delta Hat) updated and republished this content in July 2025.

Share