Welcome to our ISPOR Europe 2025 virtual booth
To help save the planet and to save you from carrying a tonne of literature around the exhibition hall, all our resources are available virtually.
Speak to our global market access, pricing, and HEOR experts:
Are you confident in your market access and pricing strategy?
Let us push the boundaries of your approach and maximise your global impact
Planning to enter the European markets?
We’ll guide you through complex reimbursement and access environments
Preparing for reimbursement and HTA negotiations?
We’ll ensure your submissions succeed at every step
Need a powerful economic proposition?
Our compelling evaluations clearly demonstrate the value of your product
Book a meeting and redeem your free hour of consultancy
Pharma and Medtech colleagues who meet our team at ISPOR Europe are offered a voucher for 1 hour of free consultancy with our experts.
To redeem this, please email evidence@petauri.com.
Spotlight on global pricing and market access strategy
A clear global pricing and market access (P&MA) strategy is essential for commercial success and appropriate uptake across markets. Our experienced team partners with clients to guide strategic decisions around positioning, evidence gaps, value, and pricing.
Using desk research, data analysis, analogue assessments, payer validation, and modelling, we deliver the insights needed to develop an evidence-based pricing and access strategy in today’s changing environment.
Our services span the full product lifecycle – from early analogue assessments and input to evidence planning, through to pricing strategy, forecasting, innovative agreements, contracting, and payer negotiation. We also offer rapid BD/in-licensing evaluations and peer-to-peer payer advisory boards.
Spotlight on digital value communication tools
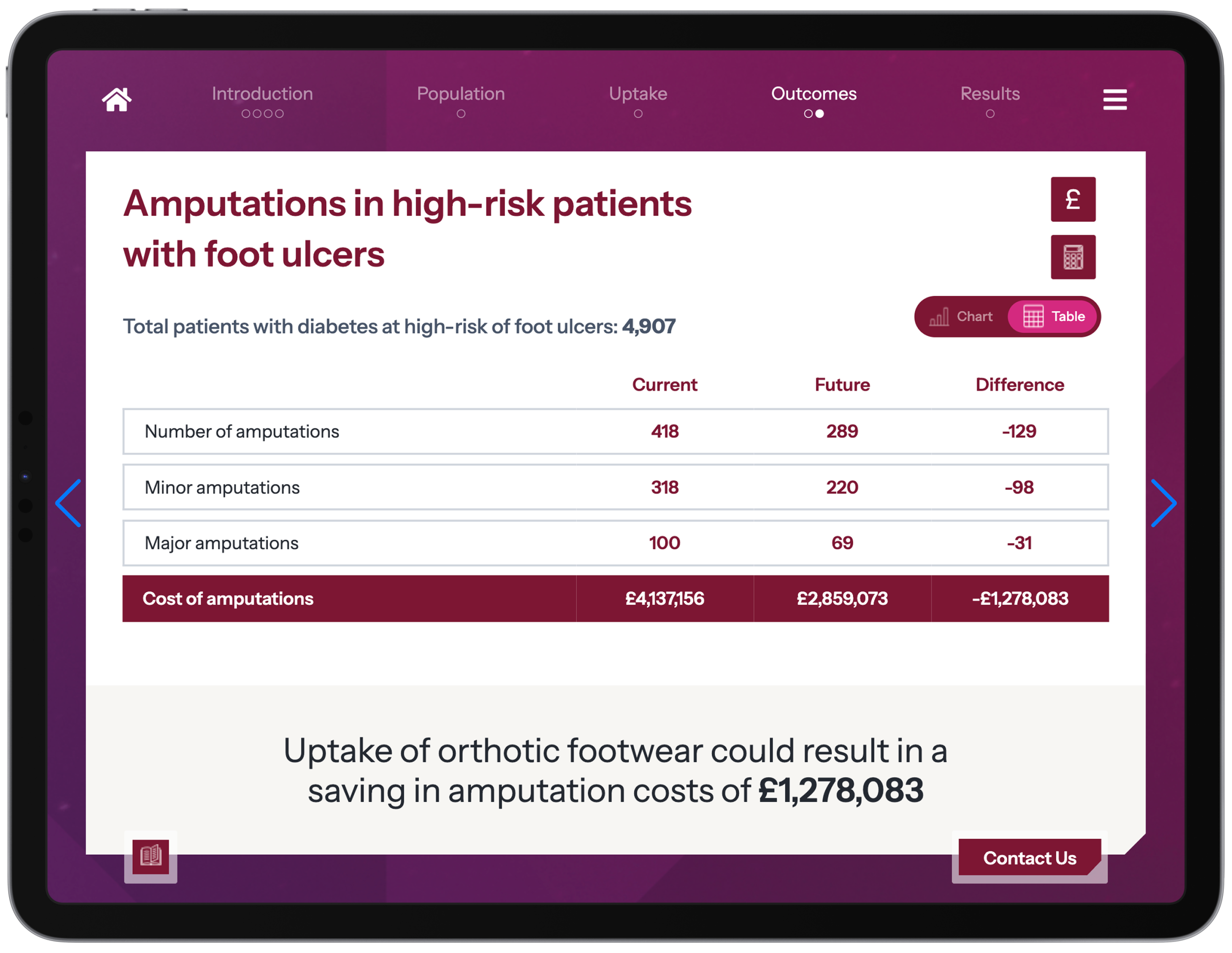
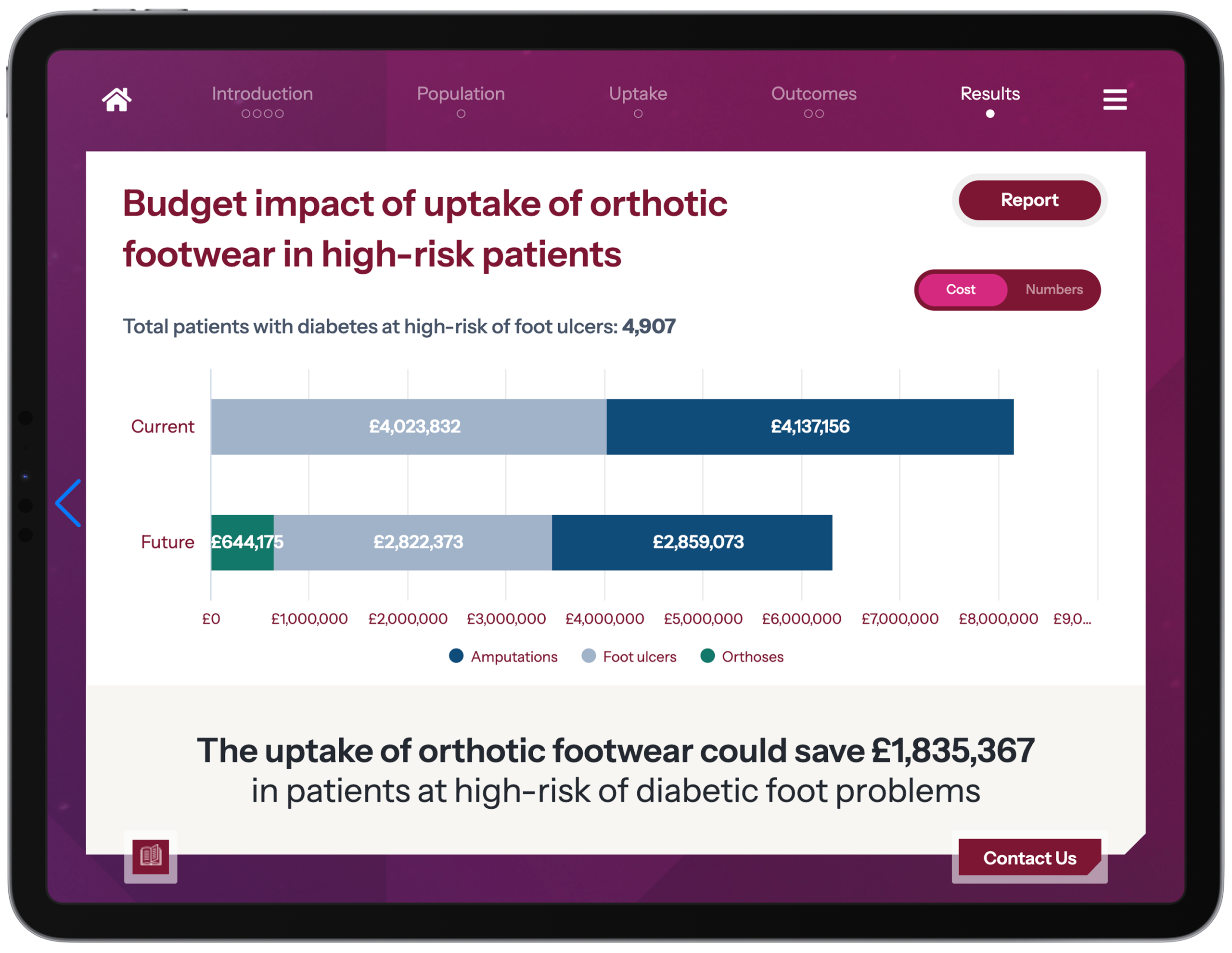
Transform your payer engagement with our interactive budget impact models and value communication toolkits, which bring your evidence and value proposition to life in a way that resonates with decision-makers across the care pathway.
We believe that creativity is key to impactful communication. Our in-house team of Veeva-accredited Developers and creative Graphic Designers ensure that, next to science, creativity is at the heart of what we do.
Email evidence@petauri.com to request a demo.
Thought leadership at ISPOR: Sessions
Our experts will feature in the following sessions at ISPOR this year. Catch their insights at:
Monday
11:45 – Ash Bullement will be speaking in the forum session: Survival at the Margins: Navigating HTA Challenges in Modern Oncology Trials
17:00 – Nick Latimer will be among the panellists for: Barriers and Enablers to the Adoption of Innovative Methods for Health Technology Assessment (HTA) Decision-Making
Tuesday
10:15 – Nick Latimer will be moderating a session on: Advancing Statistical Methods in Survival Analysis
17:00 – Nick Latimer will share his thoughts as a panellist for: Survival Analysis in the Era of Generative AI
Thought leadership at ISPOR: Poster research
Alongside our posters with clients, we have undertaken independent research in a number of exciting areas and will be presenting the following posters at ISPOR. To request a copy of a poster or to learn more about our research, please email evidence@petauri.com.
Session 1: Monday 10:30–13:30
- A decade in decision-making: Reviewing trends in STAs by NICE in different disease areas
- Bridging the gap between AI principles and practice: A comparative evaluation of AI position statements and frameworks for responsible use – Learn more
Session 2: Monday 16:00–19:00
- Examining the relationship between the NICE WTP threshold and the severity modifier
- Evaluating the role of patient-reported outcomes in Phase 4 clinical trials: Trends, applications, and implications for post-marketing surveillance
- Evolving trends in US healthcare policy: Perspectives from US formulary decision makers
Session 3: Tuesday 10:30–13:30
- Evolving payer preferences for health economic information and communication in the US
Session 4: Tuesday 16:00–19:00
- Simulated treatment comparisons in NICE technology appraisals: Frequency, trends, and implications
- Navigating large evidence bases for MTEP submissions and for the new NICE HealthTech program – Learn more
- Performance of large language model clinical data extraction by data domain: A rapid systematic review – Learn more
Session 5: Wednesday 09:00–11:30
- The role of environmental evidence in HTA: Comparative insights into global assessment frameworks – Learn more
- US PIE and AMCP dossiers: Payer preferences, best practice, and a comparison with global evidence dossiers
- The landscape of antibiotic development and regulation in the United States and Europe, alongside evolving industry incentive strategies – Learn more
- Quantifying the impact of inconsistencies in economic modelling assumptions: A case study in metastatic colorectal cancer
- Integrating patient-reported outcome measures and patient experience into health technology assessment: A path towards patient-centred decision-making – Learn more
- What is driving the increase in terminated NICE appraisals? – Learn more
- The use of real-world evidence in health technology assessment and the application of the NICE RWE framework: A review of NICE technology appraisals
- Traditional versus generative AI: A rapid systematic review assessing accuracy and efficiency of AI in title/abstract screening – Learn more